
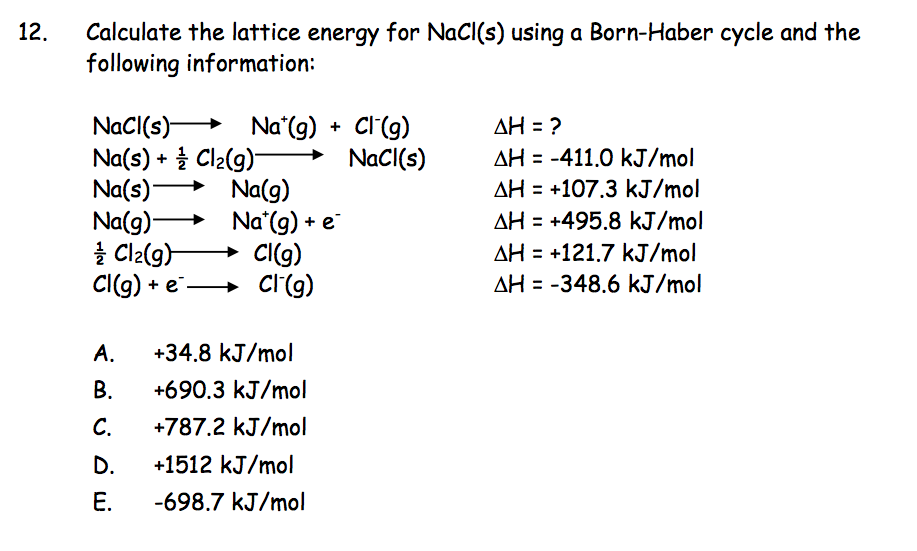
Electron Affinity is the energy released when an electron is added to a neutral atom or an ion.There are some excepts, usually due to the stability of half-filled and completely filled orbitals. In general, ionization energy increases across the periodic table from left to right, and decreases from top to bottom. This process always requires an input of energy, and thus will always have a positive value. Ionization Energy is the energy required to remove an electron from a neutral atom or an ion.There are several important concept to understand before the Born-Haber Cycle can be applied to determine the lattice energy of an ionic solid ionization energy, electron affinity, dissociation energy, sublimation energy, heat of formation, and Hess's Law. Some require such high temperatures that they decompose before they can reach a melting and/or boiling point. It is this that causes ionic solids to have such high melting and boiling points. A lot of energy is released as the oppositely charged ions interact. However, the crystalline structure allows each ion to interact with multiple oppositely charge ions, which causes a highly favorable change in the enthalpy of the system. Some might expect such an ordered structure to be less stable because the entropy of the system would be low. Lattice Energy is used to explain the stability of ionic solids. Its values are usually expressed with the units kJ/mol. As implied in the definition, this process will always be exothermic, and thus the value for lattice energy will be negative. The other definition says that lattice energy is the reverse process, meaning it is the energy released when gaseous ions bind to form an ionic solid.

This definition causes the value for the lattice energy to always be positive, since this will always be an endothermic reaction. In one definition, the lattice energy is the energy required to break apart an ionic solid and convert its component atoms into gaseous ions. Lattice Energy is a type of potential energy that may be defined in two ways.


 0 kommentar(er)
0 kommentar(er)
